Reference clinical infrastructure in Latin America
Our structure
High capacity and efficiency in clinical studies
We conduct multiple studies simultaneously with independent infrastructure, ensuring participant safety and data integrity.
Take a virtual tour
20,000 m² of dedicated infrastructure
Operational excellence at all stages of the study.
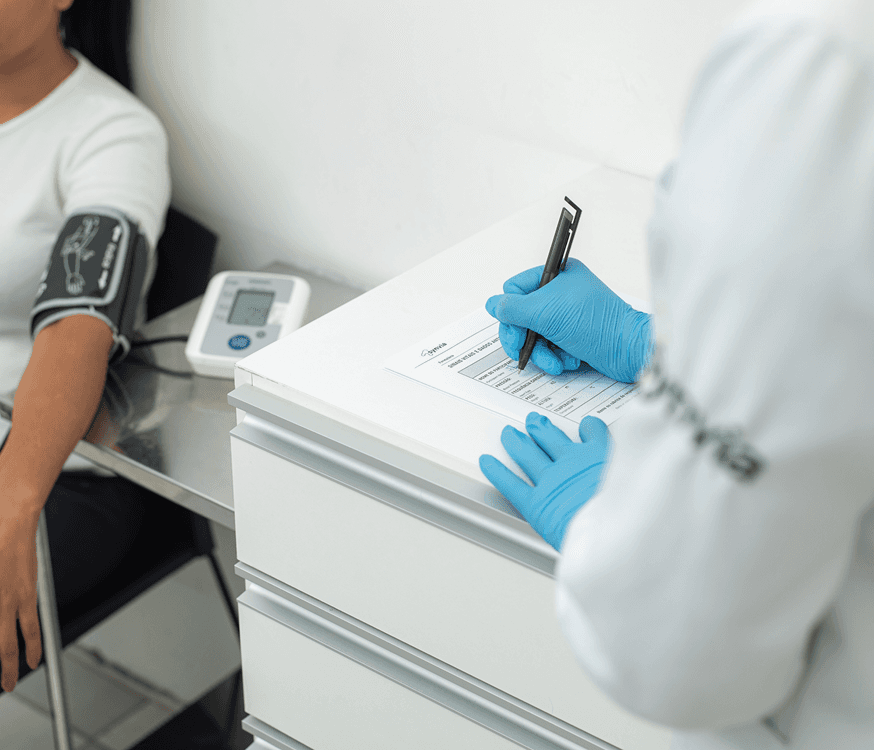
Research participant screening unit
Dedicated unit for participant recruitment (screening), with the capacity for more than 5,000 screenings per month. It has independent areas and separate flows to optimize service.
Modular Wards
and Intensive Observation Rooms
162 beds distributed across four independent wards, allowing the simultaneous conduction of different clinical studies with total operational isolation and control of variables, and rooms equipped with continuous cardiac monitoring.
Technical file and regulatory record
Area dedicated to the secure storage of physical documents, regulatory records, and critical files. It has strict access control, complete traceability, and continuous monitoring of the environment, following best documentation practices and external audit requirements.
Monitored freezers and ultra-freezers
Complete infrastructure for clinical research
Environments prepared for the comfort of participants, data security, and technical support for teams.
Integrated technology for precision and traceability
Electronic data capture and management systems ensure real-time monitoring, information integrity, and regulatory compliance.
Integrated bioanalytical laboratory with the clinical center
We have a highly qualified technical team, including specialists with master's and doctoral degrees in method development and validation.
The proximity between the clinical units and the bioanalytical laboratory ensures efficiency, traceability, and global compliance.
+100 thousand
samples/month
+500
bioanalytical methods
The largest analytical park in Latin America for mass spectrometry
Our modern facilities include state-of-the-art equipment, such as:
Validated infrastructure and global compliance
Our facilities meet international quality and safety standards, ensuring the credibility of every result delivered.