
Quality and safety
Clinical data management with cutting-edge technology and regulatory compliance
The Synvia eCRF digitizes and centralizes the collection of clinical data throughout the study — from field filling to the final database — ensuring information integrity, complete traceability, and operational efficiency, in full compliance with national and international regulatory requirements (ANVISA, ICH-GCP, EMA, and FDA).
ANVISA
ANVISA
ANVISA
ANVISA
FDA 21 CRF PART 1
FDA 21 CRF PART 1
FDA 21 CRF PART 1
FDA 21 CRF PART 1
EMA
EMA
EMA
EMA
I
I
I
I




High-performance solution that brings more precision, agility, and innovation to clinical studies.



Enhanced efficiency:
Collection and analysis of data in a simplified way
Enhanced efficiency:
Collection and analysis of data in a simplified way
Enhanced efficiency:
Collection and analysis of data in a simplified way
Enhanced efficiency:
Collection and analysis of data in a simplified way
Optimized cost:
Solution with optimized cost for the Brazilian market
Optimized cost:
Solution with optimized cost for the Brazilian market
Optimized cost:
Solution with optimized cost for the Brazilian market
Optimized cost:
Solution with optimized cost for the Brazilian market
Agile decisions:
Data updated in real time
Agile decisions:
Data updated in real time
Agile decisions:
Data updated in real time
Agile decisions:
Data updated in real time
Greater accuracy:
Automation and validations minimize human errors
Greater accuracy:
Automation and validations minimize human errors
Greater accuracy:
Automation and validations minimize human errors
Greater accuracy:
Automation and validations minimize human errors
Advanced technology:
Application of Artificial Intelligence and robust integrations
Advanced technology:
Application of Artificial Intelligence and robust integrations
Advanced technology:
Application of Artificial Intelligence and robust integrations
Advanced technology:
Application of Artificial Intelligence and robust integrations
Agile and efficient support:
Quick responses and improvements implemented promptly.
Agile and efficient support:
Quick responses and improvements implemented promptly.
Agile and efficient support:
Quick responses and improvements implemented promptly.
Agile and efficient support:
Quick responses and improvements implemented promptly.
Standard Modules
Main features
Information Security
Advanced encryption, access control, and international regulatory compliance
Information Security
Advanced encryption, access control, and international regulatory compliance
Information Security
Advanced encryption, access control, and international regulatory compliance
Anonymization of participants
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Anonymization of participants
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Anonymization of participants
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Queries and Data
Efficient query management and data consistency assurance
Queries and Data
Efficient query management and data consistency assurance
Queries and Data
Efficient query management and data consistency assurance
Forms Status
Detailed tracking of form completion
Forms Status
Detailed tracking of form completion
Forms Status
Detailed tracking of form completion
Real Time Reports
Detailed reports available in real-time for monitoring
Real Time Reports
Detailed reports available in real-time for monitoring
Real Time Reports
Detailed reports available in real-time for monitoring
Electronic Forms
Guaranteed secure, accurate, and standardized data collection
Electronic Forms
Guaranteed secure, accurate, and standardized data collection
Electronic Forms
Guaranteed secure, accurate, and standardized data collection
Source Data Verification (SDV)
Resource for managing the monitoring activities (SDV) of the study
Source Data Verification (SDV)
Resource for managing the monitoring activities (SDV) of the study
Source Data Verification (SDV)
Resource for managing the monitoring activities (SDV) of the study
Participant Status
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Participant Status
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Participant Status
Inclusion of participants in a secure and anonymous way, ensuring data protection.
User Management
Strict control of user access and their permissions (roles) within the eCRF
User Management
Strict control of user access and their permissions (roles) within the eCRF
User Management
Strict control of user access and their permissions (roles) within the eCRF
User Management
Strict control of user access and their permissions (roles) within the eCRF
Participant Status
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Source Data Verification (SDV)
Resource for managing the monitoring activities (SDV) of the study
Electronic Forms
Guarantee of secure, accurate, and standardized data collection
Real Time Reports
Detailed reports available in real-time for monitoring
Forms Status
Detailed tracking of form completion
Queries and Data
Efficient query management and data consistency assurance
Anonymization of participants
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Information Security
Advanced encryption, access control, and international regulatory compliance
User Management
Strict control of user access and their permissions (roles) within the eCRF
Participant Status
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Source Data Verification (SDV)
Resource for managing the monitoring activities (SDV) of the study
Electronic Forms
Guarantee of secure, accurate, and standardized data collection
Real Time Reports
Detailed reports available in real-time for monitoring
Forms Status
Detailed tracking of form completion
Queries and Data
Efficient query management and data consistency assurance
Anonymization of participants
Inclusion of participants in a secure and anonymous way, ensuring data protection.
Information Security
Advanced encryption, access control, and international regulatory compliance
Optional Modules
Add more features to your system
APIs
Secure and efficient integrations with external systems
Randomization
Secure automation of the participant randomization process
Medical Coding
Accurate and standardized coding of medical terms and medications through the MedDRA and WHO Drug dictionaries.
Lab Normals
Automated management of the laboratory test results of the participants
Inventory and Dispensation
Detailed and secure management of the stock and dispensing of the study medications (PSI)
Notifications
Automated communication for important alerts and reminders
APIs
Secure and efficient integrations with external systems
Randomization
Secure automation of the participant randomization process
Medical Coding
Accurate and standardized coding of medical terms and medications through the MedDRA and WHO Drug dictionaries.
Lab Normals
Automated management of the laboratory test results of the participants
Inventory and Dispensation
Detailed and secure management of the stock and dispensing of the study medications (PSI)
Notifications
Automated communication for important alerts and reminders
APIs
Secure and efficient integrations with external systems
Randomization
Secure automation of the participant randomization process
Medical Coding
Accurate and standardized coding of medical terms and medications through the MedDRA and WHO Drug dictionaries.
Lab Normals
Automated management of the laboratory test results of the participants
Inventory and Dispensation
Detailed and secure management of the stock and dispensing of the study medications (PSI)
Notifications
Automated communication for important alerts and reminders
The platform that stands out in the market and drives clinical studies
Regulatory Compliance
Regulatory Compliance
Investment
Investment
Performance
Performance
Usability
Usability
Auditability
Auditability
Flexibility and scalability
Flexibility and scalability
Implementation
Implementation
Technical support & Improvements
Technical support & Improvements
Synvia eCRF
National platform,
global standard
Fully complies with national and international standards
Fully complies with national and international standards
Fully complies with national and international standards
Fully complies with national and international standards
National reality
Investment compatible with the national reality
National reality
National reality
Optimized for exceptional performance
Optimized for exceptional performance
Optimized for exceptional performance
Optimized for exceptional performance
Friendly and intuitive interface
Friendly and intuitive interface
Friendly and intuitive interface
Friendly and intuitive interface
Controlled, tracked, and auditable record of operations
Controlled, tracked, and auditable record of operations
Controlled, tracked, and auditable record of operations
Controlled, tracked, and auditable record of operations
Highly adaptable to various and complex studies
Highly adaptable to various and complex studies
Highly adaptable to various and complex studies
Highly adaptable to various and complex studies
Quick implementation with personalized training
Quick implementation with personalized training
Quick implementation with personalized training
Quick implementation with personalized training
Agile and personalized
Agile and personalized support
Agile and personalized
Agile and personalized support
Other platforms
They may not meet all regulatory requirements
They may not meet all regulatory requirements
They may not meet all regulatory requirements
Insecurity regarding exchange rate variation
Insecurity regarding exchange rate variation
Insecurity regarding exchange rate variation
Low performance
Low performance
Low performance
Complex interfaces
Complex interfaces
Complex interfaces
Limited records
Limited records
Limited records
Restricted options
Restricted options
Restricted options
Extensive training
Extensive training
Extensive training
Bureaucratic processes and little flexibility
Bureaucratic processes and little flexibility
Bureaucratic processes and little flexibility
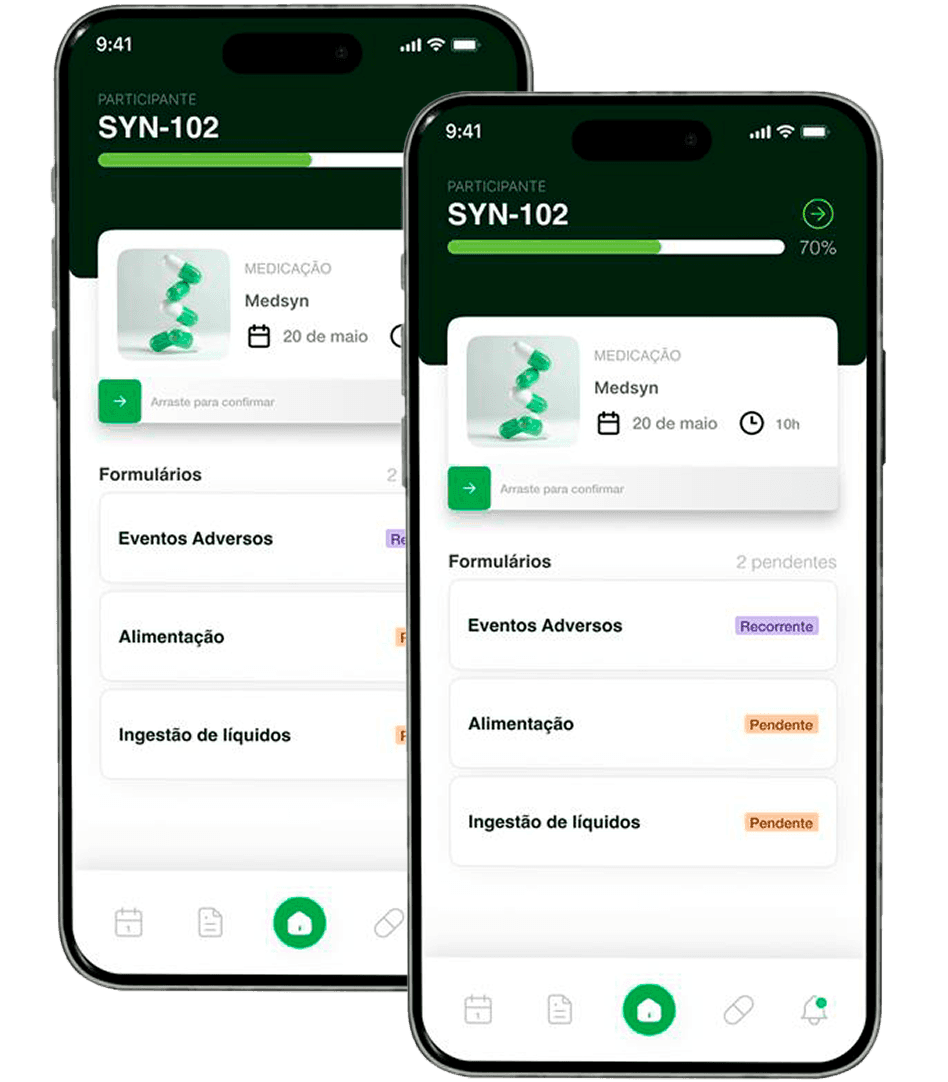



Synvia ePRO
Connect participants to clinical studies in real time – simple, safe, and regulatory compliant.
Native integration with Synvia eCRF ensuring consistency, traceability, and elimination of rework.
Native integration with Synvia eCRF ensuring consistency, traceability, and elimination of rework.
Native integration with Synvia eCRF ensuring consistency, traceability, and elimination of rework.
Native integration with Synvia eCRF ensuring consistency, traceability, and elimination of rework.
Intuitive application for the research participant, with smart reminders and notifications.
Intuitive application for the research participant, with smart reminders and notifications.
Intuitive application for the research participant, with smart reminders and notifications.
Intuitive application for the research participant, with smart reminders and notifications.
Remote and centralized monitoring for more agile and assertive decisions.
Remote and centralized monitoring for more agile and assertive decisions.
Remote and centralized monitoring for more agile and assertive decisions.
Remote and centralized monitoring for more agile and assertive decisions.
Data Management
Complete clinical data management service:
Development planning for eCRF
Development planning for eCRF
Development planning for eCRF
Development planning for eCRF
Guarantee of standardized and efficient processes that comply with regulatory requirements
Guarantee of standardized and efficient processes that comply with regulatory requirements
Guarantee of standardized and efficient processes that comply with regulatory requirements
Guarantee of standardized and efficient processes that comply with regulatory requirements
Real-time, consistent, secure, and traceable data acquisition
Real-time, consistent, secure, and traceable data acquisition
Real-time, consistent, secure, and traceable data acquisition
Real-time, consistent, secure, and traceable data acquisition





Synvia LIS
The Synvia LIS automates the complete laboratory workflow, ensuring traceability, efficiency, and proven compliance, recognized by international certifications.
Synvia LIS
The Synvia LIS automates the complete laboratory workflow, ensuring traceability, efficiency, and proven compliance, recognized by international certifications.

Synvia LIS
The Synvia LIS automates the complete laboratory workflow, ensuring traceability, efficiency, and proven compliance, recognized by international certifications.

Synvia LIS
The Synvia LIS automates the complete laboratory workflow, ensuring traceability, efficiency, and proven compliance, recognized by international certifications.

Synvia CTMS
Electronic data collection platform reported directly by participants, ideal for sponsors and research centers.
Synvia CTMS
Electronic data collection platform reported directly by participants, ideal for sponsors and research centers.

Synvia CTMS
Electronic data collection platform reported directly by participants, ideal for sponsors and research centers.

Synvia CTMS
Electronic data collection platform reported directly by participants, ideal for sponsors and research centers.

Synvia eTMF
Electronic management platform for the Trial Master File, with organization, traceability, and control of regulatory document versions, ideal for sponsors and research centers.
Synvia eTMF
Electronic management platform for the Trial Master File, with organization, traceability, and control of regulatory document versions, ideal for sponsors and research centers.

Synvia eTMF
Electronic management platform for the Trial Master File, with organization, traceability, and control of regulatory document versions, ideal for sponsors and research centers.

Synvia eTMF
Electronic management platform for the Trial Master File, with organization, traceability, and control of regulatory document versions, ideal for sponsors and research centers.

Contact
Talk to our experts
Discover how Synvia can support your studies, analyses, and projects.

Contact
Talk to our experts
Discover how Synvia can support your studies, analyses, and projects.